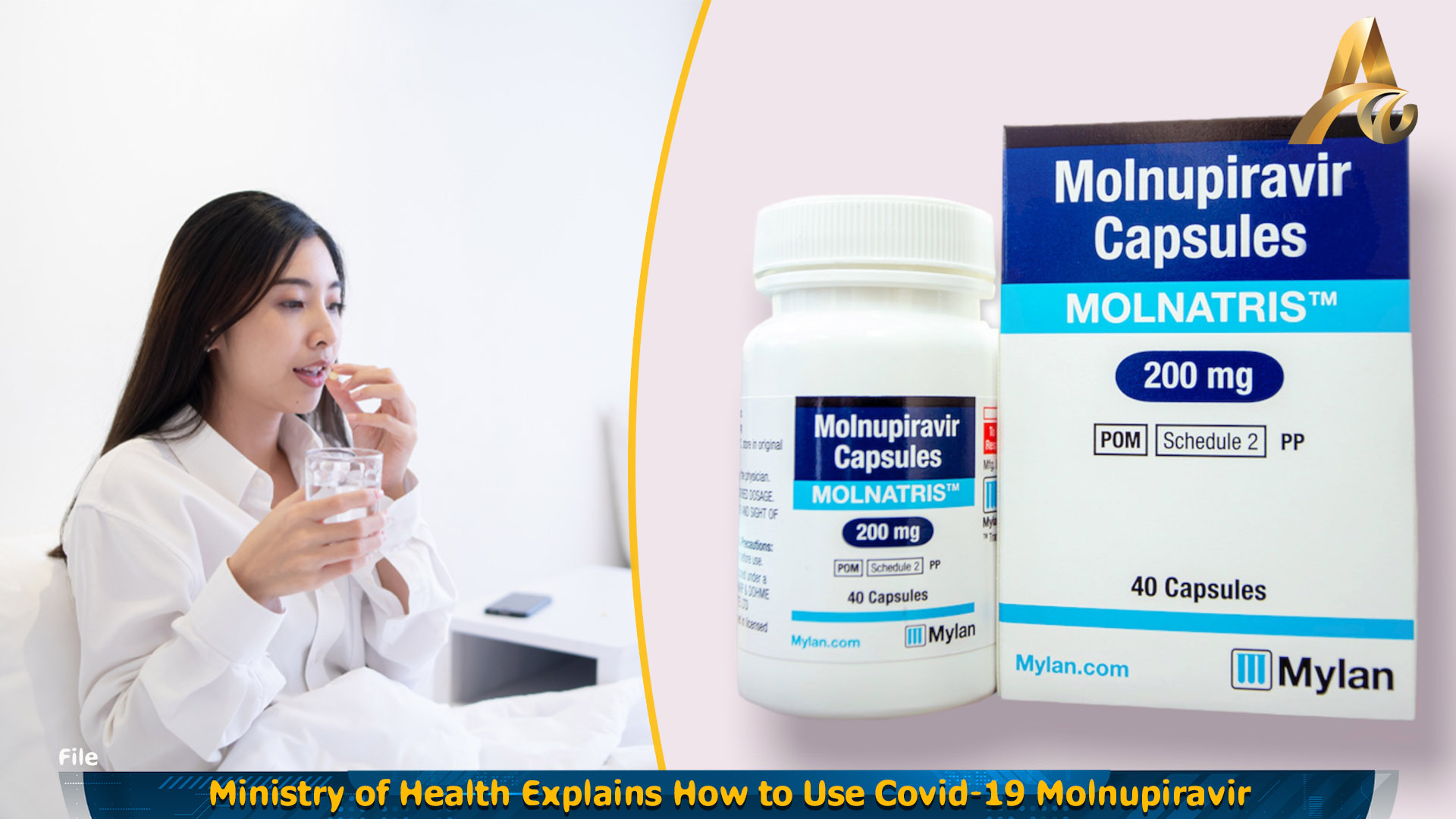
Phnom Penh: The Ministry of Health has recommended the use of Molnatris (Moltopiravapazul 200 mg) for the treatment of infectious diseases caused by Covid-19 from mild to moderate in adults aged 18 years and up.
The drug is recommended by the ministry after the person has been tested positive for the SARS-COV-2 virus, especially in people who are at risk of developing a serious illness.
For consumption, it can be taken after meals or on an empty stomach with a glass of water without chewing or grinding.
It is recommended to take 800 mg orally, equivalent to 4 tablets, taken every 12 hours for 5 consecutive days. The ministry recommends that the drug be used as soon as possible after a diagnosis of Covid-19 and within five days of onset of symptoms.
If the patient forgets to take Molnupiravir once every 10 hours, the patient should take it as soon as possible and continue to take the usual dose regularly. If the patient forgets to take the dose once more than 10 hours, the patient should not take the forgotten dose and instead take the next dose at the usual time.
"Patients should not take twice the dose to supplement the dose they forgot to take," the ministry said. Elderly, patients with impaired renal function, patients with impaired liver function do not need to adjust the dose of Molnupiravir based on age and type of disease.
For children under the age of 18, the Ministry has not yet been able to confirm safety and effectiveness.
This medicine can cause minor side effects such as diarrhea, nausea, vomiting, dizziness, headache and may have skin rashes, hives, etc. In case of an overdose of Molnupiravir, there should be monitoring of the patient's health status and immediate medical intervention as necessary.
Although there are no data on the use of Molnupiravir in pregnant women, animal studies have shown fertility poisoning. Therefore, Molnupiravir is not recommended for use during pregnancy. Women who are planning to become pregnant should use effective contraception for the duration of treatment and 4 days after the end of Molnupiravir.
For women who are breastfeeding, it is not yet known whether Molnupiravir or any of the ingredients of Molnupiravir present in human milk affect human milk production or affect breastfed infants.
Based on the side effects of Molnupiravir in infants, breastfeeding is not recommended during treatment and for 4 days after stopping using Molnupiravir.